I³LUNG
I³LUNG
I³LUNG: An EU-funded Project fostering the implementation of artificial intelligence (AI)-based personalized medical care for lung cancer patients
Recently, the European Commission launched the Horizon Europe program to promote research and innovation, in order to achieve the United Nations’ Sustainable Development Goals and boost the European Union’s competitiveness and growth. The program will run until 2027 with a budget of €95.5 billion and is open to all types of organizations including academia, industry, and charities. Of this amount, roughly €8.33 billion will be allotted to the “Health cluster”, focused on a variety of topics among which staying healthy in a rapidly changed society.
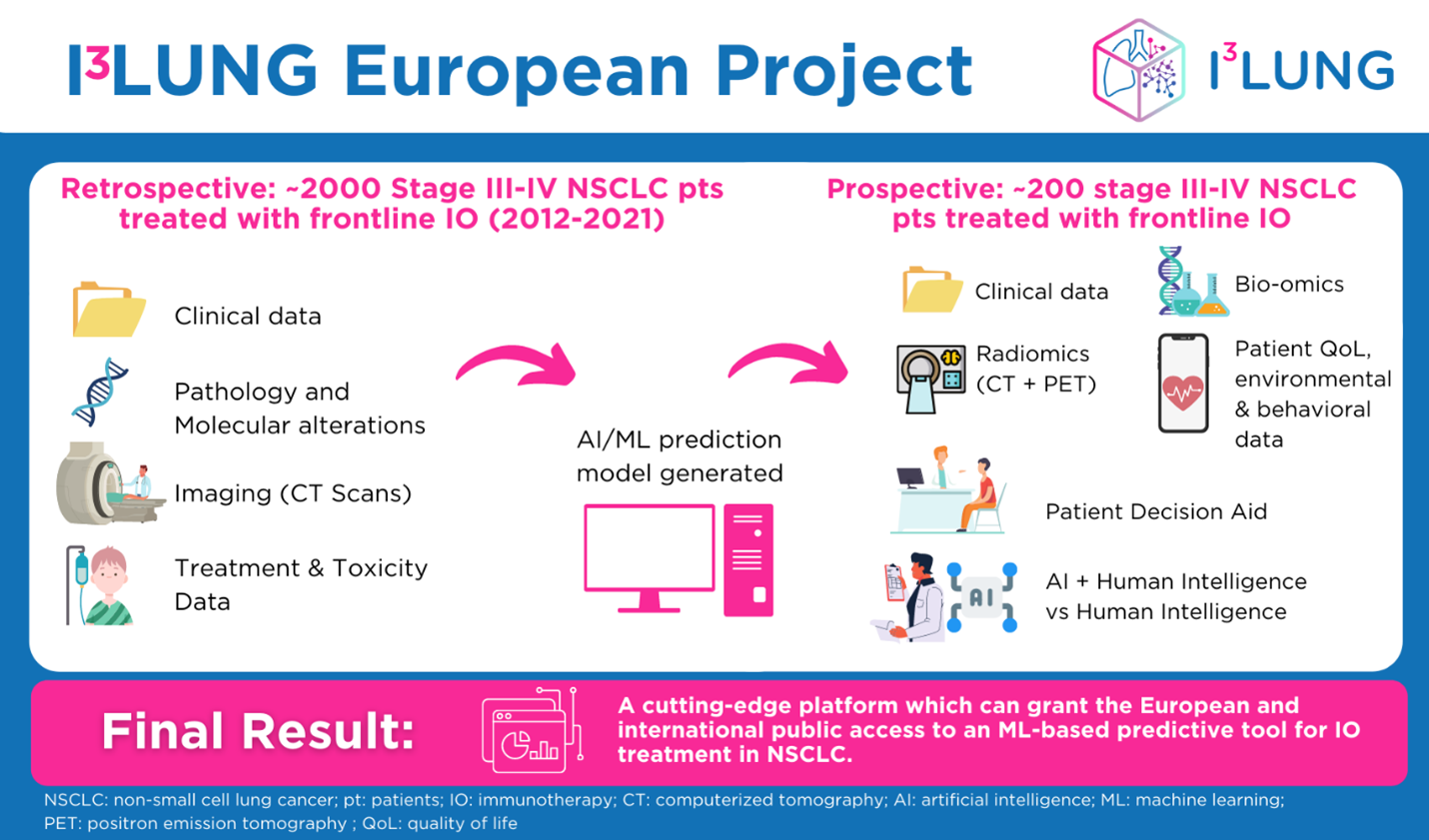
Submitted as an answer to a call for new projects with the goal to ensure access to innovative, sustainable, and high-quality health care, I³LUNG* is a research initiative that aims to create a cutting-edge decision-making tool to aid both clinicians and patients in selecting the best lung cancer treatment plan, tailored to the specific needs and situation of each individual patient. To accomplish this, the project will use artificial intelligence (AI), in particular deep and machine learning methodologies (DL and ML) to analyze a wide range of information such as baseline clinical features, radiomics, and available biological characteristics of the tumor. All these data will come from the retrospective analysis of 2,000 patients from multiple centers, along with the enrolment of 200 new patients in a prospective study for the generation of new biological multiomic data, such as: tumor mutational burden, immune circulating profiling, digital pathology, gut microbiome, radiomics and other multi-omics approaches. In parallel, a psychological study will also be conducted and will integrate the aspect of the patients’ experiences and preferences as an input in the development of a co-decision aid tool. Six outstanding clinical cancer centers located from all over the world (Italy, Germany, Greece, Spain, Israel, USA) are involved in these data collection processes.
The consortium has thought out and developed this project to address the primary unmet clinical need in the field of non-small cell lung cancer (NSCLC), which is the lack of biomarkers predicting the response of affected patients to immunotherapy (IO)-based treatments. Lung cancer was the leading cause for cancer deaths in men and the second for women in 2020, with 370000 deaths in Europe alone. Thanks to the advent of IO, the way of treating these patients has been revolutionized, positioning it as the first-line therapy for metastatic NSCLC (mNSCLC) tumors lacking a targetable driver mutation, and is used either as monotherapy or in combination with standard chemotherapy. However, only 30 to 50% of patients experience a long-lasting response. Indeed, to this day, programmed Death-Ligand 1 (PD-L1) still remains the only biomarker with a satisfactory level of prediction of a patient’s outcome to IO. The fact that its predicative performance is less than ideal, however, highlights the desperate need for more comprehensive and more precise and effective molecular analyses in this setting of patients. The latter are what this project aims at discovering and implementing.
In recent years, the explosion of AI, ML, DL and the new AI subfield of explainable Trustworthy AI has created the highly exciting opportunity to use a brand new set of tools to assess the vast amounts of data generated from clinical trials and translational researches. The I³LUNG project plans to develop a European – and beyond – platform which will capitalize on these new tools to cope with the complexity of available data on lung cancer biomarkers. By synthesizing and correlating the information from multiple sources of biological and clinical information, it will thereby help in developing a set of methods that will in turn enable the project’s technical partners to construct a novel AI tool which can provide individualized predictions for the efficacy of IO in specific patients, and offer suggestions for care pathways, in coordination with all involved actors (patients, oncologists, researchers) and their human intelligence. This individualized patient selection plan will help reduce the economic burden for patients and, in the longer term, for healthcare systems, and first and foremost it will improve the outcomes of therapy for patients by better matching them and their specific situation to different available treatments.
This challenging objective will be a collaborative effort, which will include 16 partners in the I3LUNG consortium. Some among the best European and International organizations will join forces for this ambitious project: Fondazione IRCCS Istituto Nazionale dei Tumori (INT, Milan, Italy) is the coordinator of the Consortium with its PI Dr. Arsela Prelaj, Politecnico di Milano (POLIMI, Milan, Italy), Istituto di Ricerche Farmacologiche Mario Negri (IRFMN, Milan, Italy), Istituto Europeo di Oncologia (IEO; Milan, Italy), ML Cube (Milan, Italy), LungenClinic Grosshansdorf GmbH (GHD, Grosshansdorf, Germany), Universitaetsklinikum Hamburg-Eppendorf (UKE, Hamburg, Germany), ), Vall d’Hebron Institute of Oncology (VHIO, Barcelona, Spain), Medica Scientia Innovation Research (MEDSIR, Barcelona, Spain & New Jersey, USA), Metropolitan Hospital (MH, Pireas, Greece), Shaare Zedek Medical Center (SZMC, Jerusalem, Israel), Katholieke Universiteit Leuven (KUL, Leuven, Belgium), The Swedish Institute for Health Economics (IHE, Lund, Sweden), The University of Chicago (UOC, Chicago, USA), Aalborg Universitet (AAU, Aalborg, Denmark), Lung Cancer Europe (LUCE, Bern, Switzerland).

I³LUNG and its partners will have a timeframe of 5 years and a €10M budget to turn their project’s hypothesis in a tangible tool and a new clinical reality. To our knowledge, I3LUNG is the first platform enrolling such an important number of patients in both a retrospective (2,000) and prospective (200) manner including such a diversity of multiomic data, arising as an innovative and promising technology to provide an answer to the unmet clinical need of translational research data integration and AI use.
We are excited to start this project which is envisioned to both generate novel therapeutic guidelines for clinical practice in lung cancer and support the growth of digital diagnostic tools in the European industry. Harnessing the power of AI and ML could change the way cancer is treated and push the standard of care towards a more personalized approach for each individual cancer patient. If successful, the approach presented in I³LUNG could in the near future justify an initial pilot study which could expand on the project and attempt to apply the tools and methodologies developed to all the cancer types for which patients are candidate to IO in daily practice.
We cannot wait to be part of developing a tool that could revolutionize cancer care!
IHE participates as the health economic partner in the project.
Don’t hesitate to contact IHE at ihe@ihe.se for further information on the I³LUNG European Project.
Interview with Michael Willis, Research Director at IHE
News & Publications from the project
February, 2023
Article published in Clinical Lung Cancer
The EU-funded I3LUNG Project: Integrative Science, Intelligent Data Platform for Individualized LUNG Cancer Care With Immunotherapy
April, 2023
Poster presented by IHE at SHEA 2023 (PDF)
June, 2023
Partners Meet in Barcelona to Advance the Application of AI to Lung Cancer
June, 2024
Michael Willis, research director at IHE, attended the third annual meeting for I3LUNG. In connection with the annual meeting, a 2-day conference was also organized on the subject of Artificial Intelligence in Oncology: Applications and challenges. Michael Willis was one of the participants and gave a presentation on the Ethical and Regulatory Environment of AI in Healthcare and the Impact to Patients from a Health Economics perspective.
September, 2024
Article published in Journal of Managed Care & Specialty Pharmacy
Budget Impact Models for Lung Cancer Interventions: A Systematic Literature Review
January, 2025
Article published in Journal of Managed Care & Specialty Pharmacy
Cost-effectiveness models of non–small cell lung cancer: A systematic literature review
June, 2025
The I3LUNG Consortium has gathered for the Annual Meeting, this year held on Corfu, Greece and Michael Willis, Research Director, from IHE participates at the meeting June 13. IHE participates as the health economic partner in the project. The event brings together all the international collaborators of the I3LUNG project to share the current status and progress made in this project in Artificial Intelligence (AI) applied to lung cancer and align on the way forward.
I3LUNG leads the way towards personalized oncology through artificial intelligence (pdf)
*Funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or HaDEA. Neither the European Union nor the granting authority can be held responsible for them.



